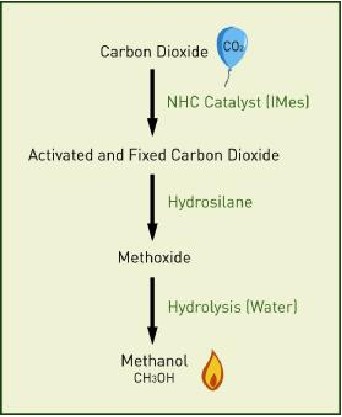
 The carbon dioxide reduction is efficiently catalyzed by NHCs even at room temperature. Then hydrosilane provides hydrogen, which bonds with carbon dioxide in a reduction reaction.
The carbon dioxide reduction is efficiently catalyzed by NHCs even at room temperature. Then hydrosilane provides hydrogen, which bonds with carbon dioxide in a reduction reaction.Yugen Zhang, Ph.D., team leader at the Agency for Science, Technology and Research in Singapore announced through a paper in the international chemistry journal Angewandte Chemie International Edition, where it was designated a “Hot Paper.” that the team activated carbon dioxide in a mild and non-toxic process to produce methanol, by using organocatalysts. The journal designated the work as a “Hot Paper.”
The team made the carbon dioxide react by using N-heterocyclic carbenes (NHCs), their novel organocatalyst. Compared to heavy metal catalysts that usually contain toxic and unstable components, NHCs are stable, even in the presence of oxygen. That makes reactions with NHCs and carbon dioxide possible under mild conditions in dry air.
Hydrosilane, a combination of silica and hydrogen, is added to the NHC-activated carbon dioxide, and the product of this reaction is transformed into methanol by adding water through hydrolysis.
The research would be very useful breakthrough to convert global warming gas to usable product.This method can be very useful in fossil fuel like coal for power millions of tons of carbon dioxide send into atmosphere can be converted useful product-methanol.
The press release explains that organocatalysts are catalysts, comprised of non-metallic elements, found in organic compounds. NHCs such as IMes (1,3-bis-(2,4,6 trimethylphenyl)imidazolylidene) are a form of organocatalysts that are stable and easily stored. They do not contain toxic heavy metals and can be produced easily without high costs.
The team showed that only a small amount of NHC is required to induce carbon dioxide activity in a reaction. Siti Nurhanna Riduan, IBN Senior Lab Officer said, “NHCs have shown tremendous potential for activating and fixing carbon dioxide. Our work can contribute towards transforming excess carbon dioxide in the environment into useful products such as methanol.
Zhang explained the process, “Hydrosilane provides hydrogen, which bonds with carbon dioxide in a reduction reaction. This carbon dioxide reduction is efficiently catalyzed by NHCs even at room temperature. Methanol can be easily obtained from the product of the carbon dioxide reaction. Our previous research on NHCs has demonstrated their multiple applications as powerful antioxidants to fight degenerative diseases, and as effective catalysts to transform sugars into an alternative energy source. We have now shown that NHCs can also be applied successfully to the conversion of carbon dioxide into methanol, helping to unleash the potential of this highly abundant gas.”
It’s a big breakthrough, as previous efforts to reduce carbon dioxide to more useful products have required more energy input and a much longer reaction time. Previous efforts also required transition metal catalysts, which are both unstable in oxygen and expensive.
It’s not a slam-dunk into commercial use just yet, though. Ongoing research at IBN aims to find cheap alternatives for the hydrosilane reagent so that the production of methanol can be even more cost-effective for mass industrial production.
It certainly does change the perspective on the CO2 output at any facility with a noteworthy volume of CO2 available. In the U.S. for example a power plant having used the base fuel could have a means to use the CO2 effluent for supplying a methanol production substituting a hard investment with a sequestration of CO2 expense compared to an investment that generates an income.
Yet these kinds of things will be subjected to the dreadful uncertainty coming from the new U.S. EPA designation of CO2 as a pollutant. Delays are now an absolute certainty with uncertainty being the new watchword. One might think that never has government done such a dumb thing. One would think that everyone would want to endlessly recycle CO2 along with everything else on the planet and open the door wide to reducing fossil fuel use and increase available fuel and energy supplies.
Old hands at life in regulated economies know better. The EPA getting into the CO2 business is a disaster, and one of the first victims will be this caliber of innovation and creativity. Whether at the investor level, across the planetary health range or the competition in the costs of fuels are in for lots more delay, innovation stifling, and simply bureaucratic interference. But it could become a boon to those countries with enough intelligence to bypass the global warming hysteria and produce fuel with what’s at hand.
The economic framework aside this kind of technology could, if reason overtakes hysteria, generate a great deal of methanol fuel product. Methanol is good stuff, hydrogen rich and tolerable in safety and easy to transport. It’s a potent fuel source for both current combustion technology and the likely fuel cells to come. It will be interesting to see the numbers when the fossil fuel users see the income potential from using the CO2 effluent for fuel product. One hopes the environment crowd understands the importance of reusing the CO2 again.”
No comments:
Post a Comment